
Membrane Reactors for Hydrogen Production
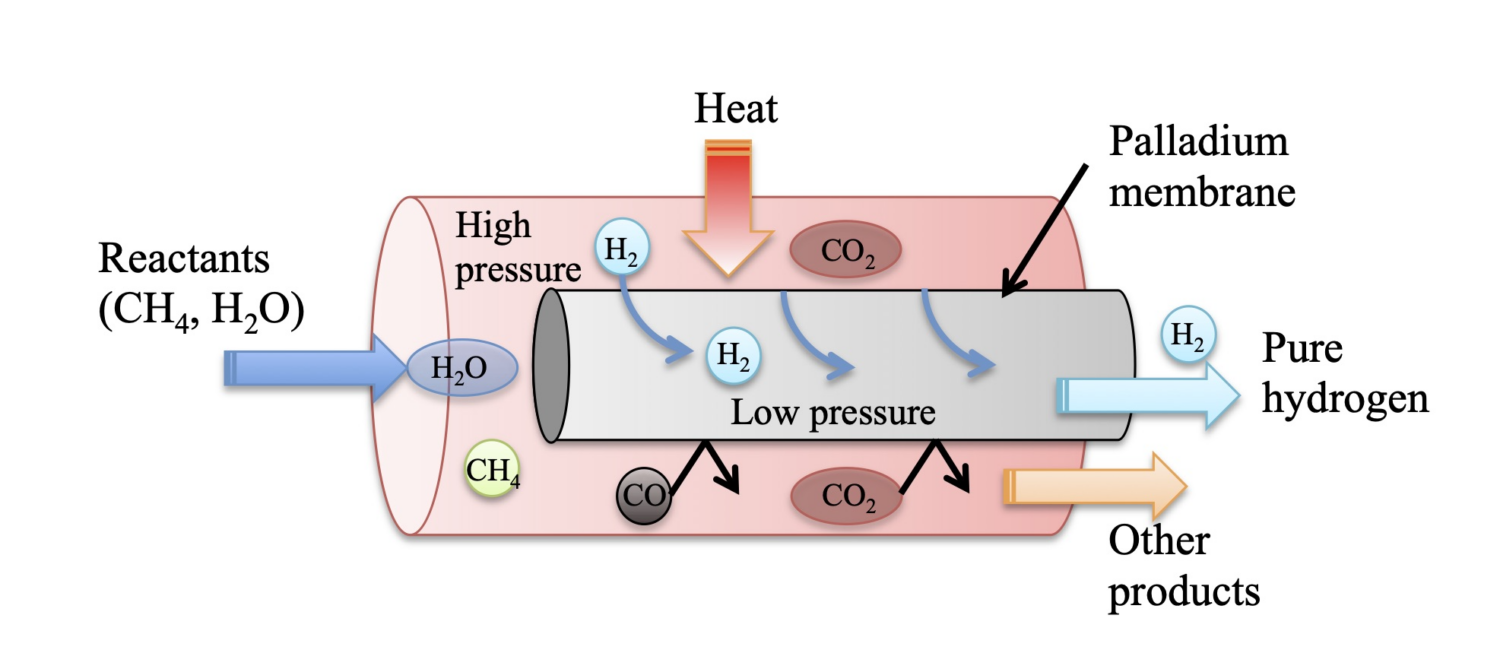
Palladium membranes are promising as means for process intensification of hydrogen production by integrating the membranes in reactors, enabling in-situ separation of hydrogen from reactions such as reforming, dehydrogenation, water-gas-shift (WGS) and more. The most important design parameter of such membrane reactors and separators is the permeance of hydrogen through the dense palladium membrane, which largely determines the performance of these units.
The objective of this research program is to contribute significantly to the understanding of surface reactions on Pd and Pd-alloy membranes as relevant to simple separators and membrane reactors employing such membranes. Specifically, it is desired to understand and demonstrate how these surface reactions impact permeance inhibition under conditions prevalent in Pd-membrane reactors for key chemical processes.
Furthermore, we investigate micro-kinetic mechanisms for reactions on Pd-membrane surfaces, and incorporate these in transient mathematical models. In-situ DRIFTS technique is implemented for probing the micro-kinetic mechanisms. These models are then used to investigate the optimal dynamic operation of such membrane reactors, with the goal to minimize inhibition and improve overall performance.
This research is funded by the Israel Science Foundation.
Membrane Reactors for CO2 Utilization
Carbon dioxide (CO2) emissions from the combustion of fossil fuels and other chemical processes (such as natural gas reforming for hydrogen production) is the key contributor to climate change and the global warming phenomenon. CO2 utilization is a sustainable approach to mitigate this harmful impact, as it leads to a sustainable material cycle (assuming the energy for the process originates from renewable sources). Highly concentrated CO2 can be used to synthesize many valuable chemicals and fuels as a C1 source.
One of the main reaction routes for CO2 utilization is the reverse water gas shift (rWGS), Eq. (1). The DRM reaction uses a fossil fuel as one of the reactants (methane, the main component of natural gas), which makes this route less attractive from a sustainability point of view. Alternatively, rWGS uses hydrogen, which can be derived from renewable sources (solar or hydrothermal electro-splitting of water).
CO2 + H2 ⇔ CO + H2O ∆H0 = 41.2kJ/mol (1)
Fischer Tropsch synthesis (FTS) is a very well known catalytic process to produce hydrocarbons from a mixture of CO and H2 (syngas). Eq. (2) is the main reaction producing paraffins, however olefins and oxygenates may also form in small concentration.
nCO + (2n + 1)H2 → nH2O + CnH2n+2 ∆H0 ≈ −165 kJ/mol (2)
Thus, the main acceptable route to produce renewable fuels (sometimes referred to as e-fuels) includes three main reaction steps. Green power generated on site (e.g. in a hydroelectric power station, or from photovoltaic cells) generates hydrogen and oxygen from water by electrolysis. In the next step the hydrogen reacts with CO2 according to the rWGS reaction, Eq. (1), to eventually form long-chain hydrocarbon compounds according to the exothermic FTS reaction, Eq. (2), in the third reaction step.
In these two process steps, water vapor (steam) are formed, which limit the conversion of the rWGS reaction due to thermodynamic equilibrium, and lead to catalyst deactivation in the FTS step. These challenges hinder the commercialization of CO2 utilization processes. A promising novel approach to address these challenges, is to conduct these reaction steps in membrane reactors utilizing high temperature hydrophilic membranes. These membranes are capable of separating the steam in-situ with high selectivities, lower the steam partial pressure in the reactor, and thus overcome the equilibrium limitations and mitigate catalyst deactivation of the traditional reactors.
Coupling Endothermic Reactions of Fuel for Cooling in Scramjet Engines
Thermal management in scramjet engines is a significant challenge. A promising cooling strategy is to utilize the fuel as the primary coolant, through endothermic reactions. This approach can also improve the combustion of the fuel and lead to higher temperatures and thus higher Mach numbers.
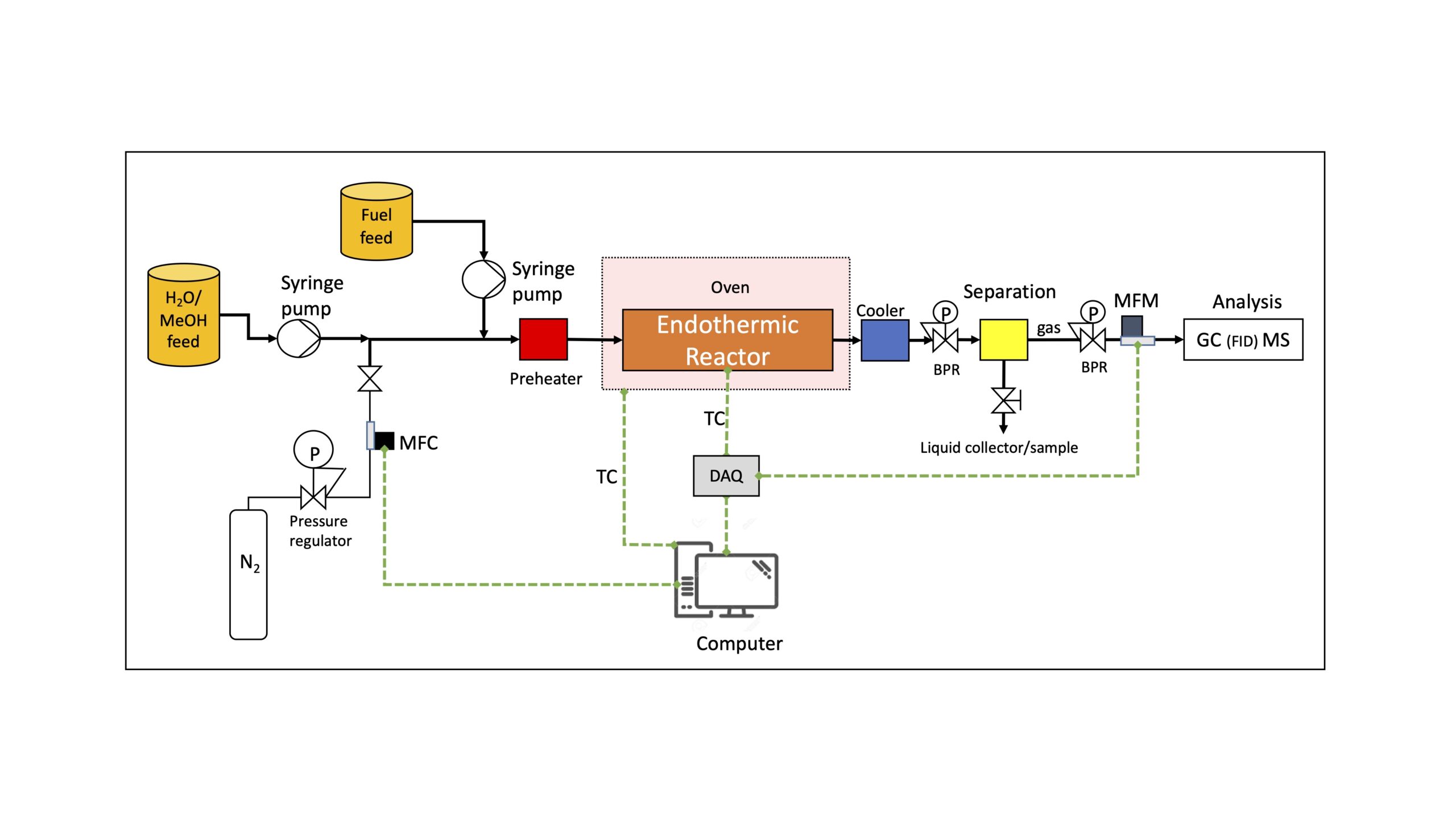
Rafael and the PI lab are leveraging the expertise of both research groups to methodologically study endothermic cooling strategies and their integration with the engine. The research program is focused on modeling jet fuels catalytic cracking, quantifying coke formation and deactivation rates, and investigating the use of alternative reactions and fuels as means of on-board cooling. See PFD of the experimental system below. The coupling (i.e. integration) of the cooling system to the combustion chamber is rigorously analyzed. We develop a computational tool for system design and optimization. An optimal system may include different sub-systems of endothermic reactors in series or parallel, operating at different conditions.
The research is funded by the Pazy Foundation.